
INTERACTIVE EBOOK
Explore Transformative Real World Data Sources
Leverage RWE to cut research costs, improve insights, and more
When you combine the growing acceptance of real world data (RWD) with challenges in the pharma industry – such as the need to accelerate speed to market, conduct studies efficiently, and secure payer approvals – the value of leveraging RWD and real world evidence (RWE) increases exponentially.
Do you and your stakeholders truly know when, where, why, and how RWD can be leveraged throughout the drug development lifecycle?
This interactive eBook highlights various sources of RWD in the United States, including what types of patient-level data they contain, and what questions they are best-suited to answer. Case examples are included that describe how RWD and analytic methods were utilized to answer key client questions spanning multiple therapeutic areas such as oncology, autoimmune diseases, migraines, etc.
Real World Evidence. Real Confidence. Real Results.
Download the interactive eBook today
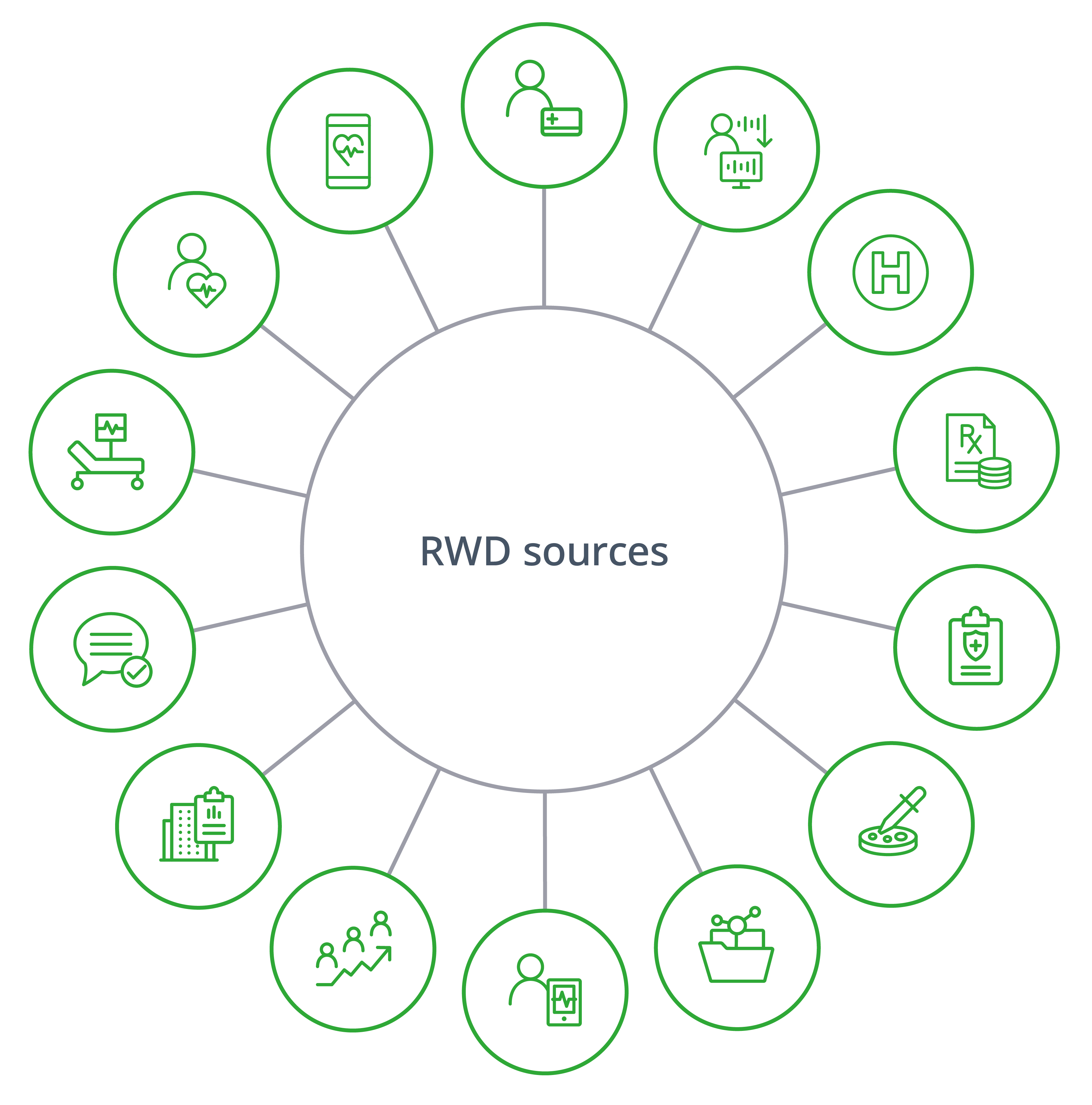
Tapping into a Wide Range of RWD Sources
A breadth of quality data is needed to design and implement effective studies. The interactive eBook will highlight a subset of different IQVIA sources that can be linked to both internal and external data sources to generate even richer insights.
Real World Data: What, When, Where, and Why?
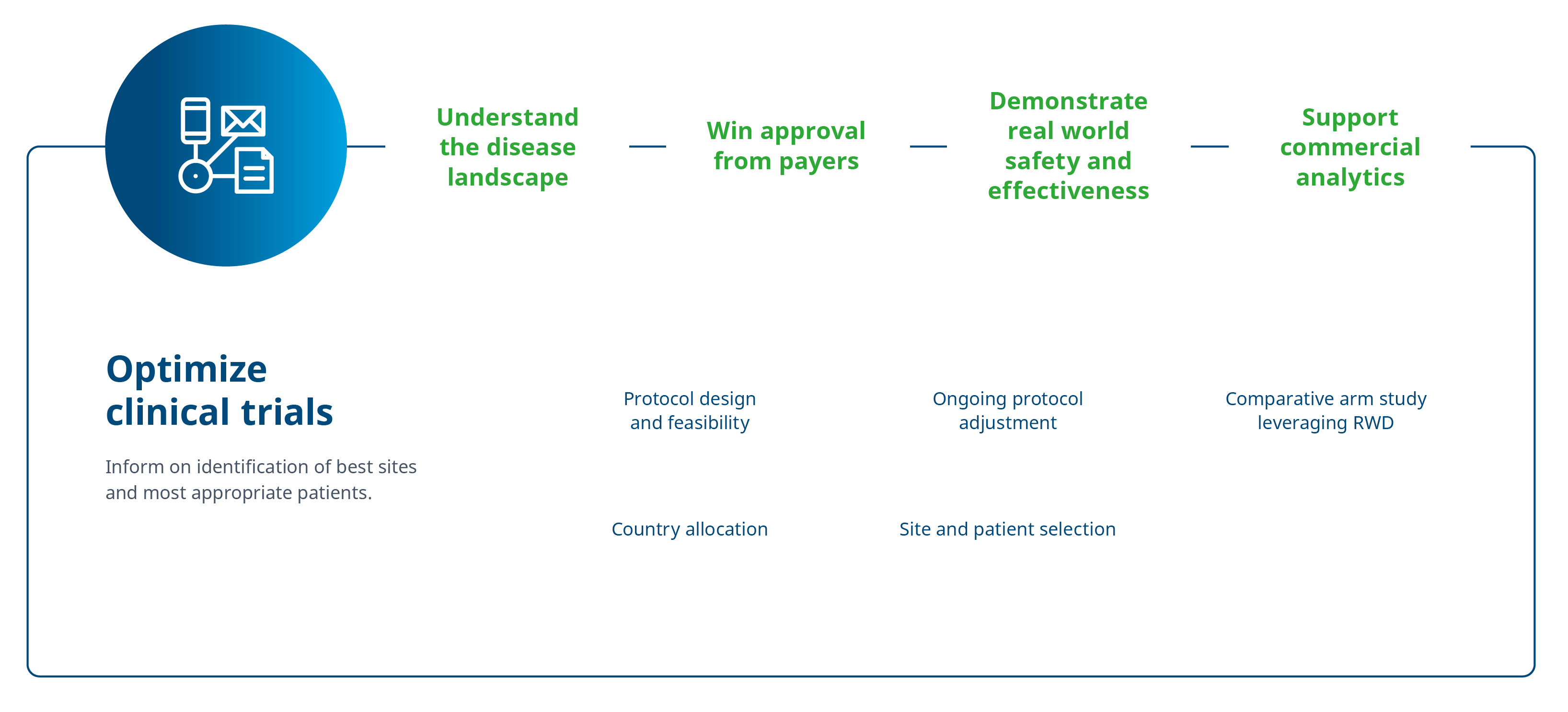
The need for real world data emerges at virtually every stage of a product’s lifecycle – it’s dynamic.
VIDEO: Real World Evidence Speaks Volumes
RWE acceptance and use continues to grow. Watch this brief video and learn how IQVIA customers have leveraged RWE to achieve their goals while challenging the status quo.
Related Content You May Enjoy

Regulators Want RWE
RWE delivers added value to the regulatory submission review process.

IQVIA PharMetrics® Plus
Comprehensive real world data on commercially insured patients in the U.S.

Copyright © 2025 IQVIA Holdings Inc. and its affiliates. All rights reserved. Privacy Statement