

IQVIA REGULATORY INTELLIGENCE
Your window to regulatory requirements across the globe
The regulatory process is challenging for any pharmaceutical or medical device. Not only do requirements differ from country to country, but they’re constantly changing too. Understanding and keeping up to date with relevant regulations can feel impossible.
IQVIA Regulatory Intelligence makes staying on top of global regulatory requirements possible, by bringing together regulatory insights and real-time updates from national authorities across the globe.
IQVIA Regulatory Intelligence is an online database that provides access to regulatory requirements for human drugs and biologics, and/or medical devices and IVDs, for over 110 countries, regions and international organizations.
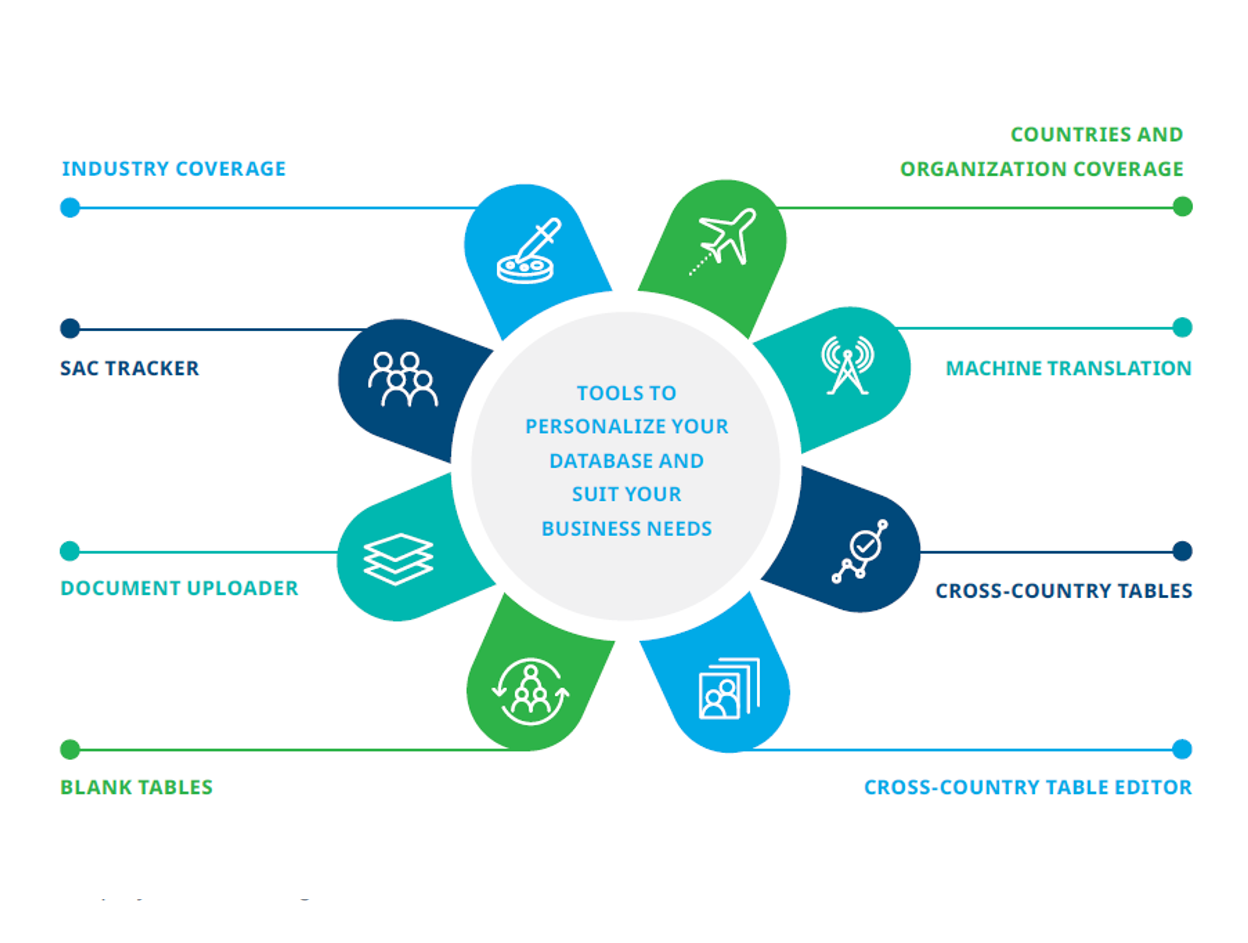
Your database can be tailored to your organization by utilizing various tools:
Want to find out more about how IQVIA Regulatory Intelligence can help you? Fill in the form to find out more about how IQVIA Regulatory Inteligence can help you or request to be contacted.

Copyright © 2026 IQVIA Holdings Inc. and its affiliates. All rights reserved. Privacy Statement