
Regulatory Affairs and Pharmacovigilance Services
IQVIA welcomes the opportunity to provide you support on Regulatory Affairs and Pharmacovigilance activities in different areas.
Find out more and download the brochures of our services. We look forward to hearing from you!
Regulatory Services

Strategic and operational support in all phases of registration for national and European procedures from the preparation of the dossier to the granting of MA, Life cycle management of medicinal products and support in the drafting and revision of Standard Operating Procedures.
Pharmacovigilance Services

Strategic and operational support in Drug Safety throughout the entire product life cycle ensuring high-quality standards, from pre-marketing pharmacovigilance activities to routine post-marketing activities.
Medical Scientific Information and Medical Compliance

Support for regulatory requirements in promotional activities like congresses and promotional materials, management of accreditations of Sales Representatives, assumption of responsibility of Scientific Service, internal audit activities and trainings.
Conferences and Meetings

Evaluation of the sponsorship of conference events, activities for managing contacts with organizational secretariats and providers, assumption of the role of Representative for Conferences and Congresses and management of AIFA authorization procedures from the request for authorization to the final accounting of event expenses.
Medical Device
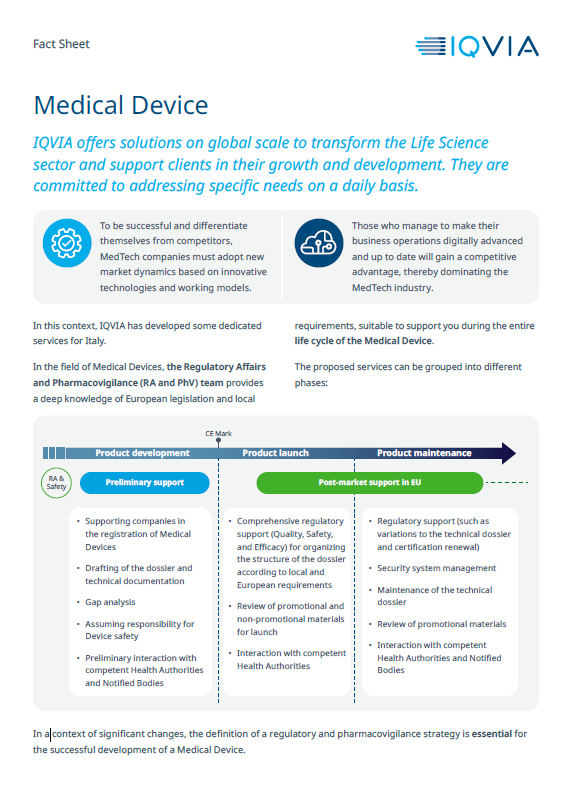
Preliminary regulatory assessment, support in the analysis of the new regulatory requirements but also in the activities necessary for the launch and maintenance of the product on the market.
Due Diligence & GAP Analysis

Support for Due Diligence and Gap Analysis of medicinal dossiers, in-depth analysis of pre-clinical, clinical and quality documentation to assess compliance with the latest regulatory requirements, worldwide assessments for different types of products.
Start-Up

Support during the various life stages of a medicinal product from registration to the entire product life cycle, ensuring compliance with European and local requirements for placing on the market and registering on the platforms required by law in the role of MAH.
Regulatory Update e Press Review

Daily or weekly newsletter with a collection and a complete overview of regulatory updates and news published on the websites of the Health Authorities and pharmaceutical magazines.
If you want to get in touch with us, please leave your contact information here and we will contact you as soon as possible:
Contact us:
Federica Santagostino
Regulatory & PhV Engagement Manager IQVIA
IQVIA Solutions Italy S.r.l.
Via Fabio Filzi, 29
20124 – Milano – Italy
