Impact of RWE on HTA Decision-making
With clinical innovation accelerating, more drugs are obtaining marketing authorization for narrowly defined patient populations and early-stage disease. This can pose challenges in clinical evidence development, as fewer patients may be available for recruitment and generating mature outcomes can be prohibitively long.
Real-world evidence (RWE) has the potential to fill this gap by complementing and supplementing clinical trial evidence, as well as reducing health technology assessment (HTA) body uncertainties that can delay reimbursement decisions. As the momentum around RWE use strengthens, examples emerge where it has contributed to positive reimbursement decisions and therefore patient access to innovative therapies. However, acceptance of RWE in HTA evaluations and its impact on HTA outcomes remains limited and highly variable among countries.
This report explores the use of RWE in HTA evaluations to inform factors that are critical to the final pricing and reimbursement decision. It presents an overview of the current role of RWE in oncology HTAs, where unmet need and obstacles to developing clinical evidence are particularly high. The report then explores key drivers and barriers of historic RWE acceptance, which builds upon work presented at ISPOR Europe 2022 of 20 examples in oncology where RWE contributed to a positive HTA outcome. Considering the value of RWE in overcoming payer uncertainties and allowing for faster patient access, the report concludes with a discussion of initiatives and strategies to standardize and optimize the way RWE is evaluated across Europe.
Some key findings:
Please submit the below form to download
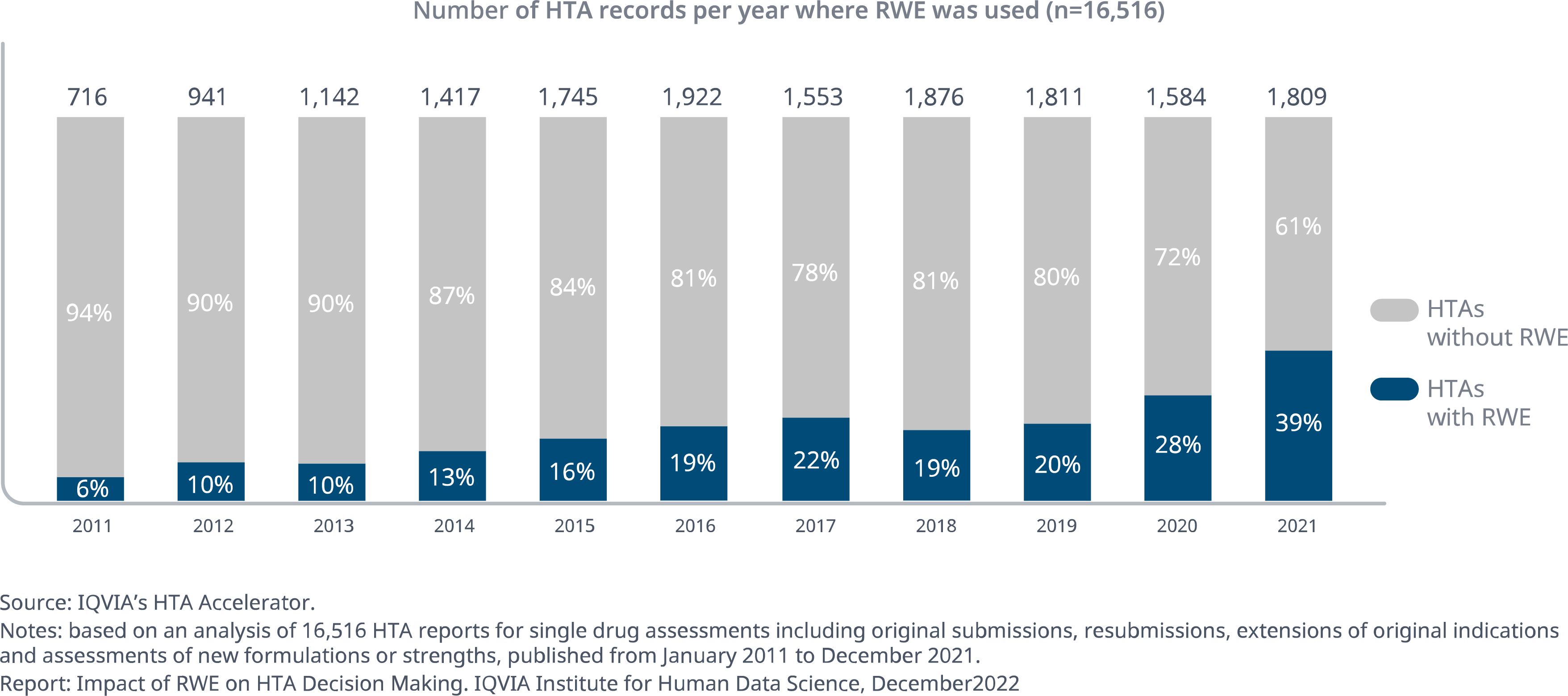
- The use of RWE as a component of submissions in HTAs has seen a marked increase in recent years.
- According to an IQVIA analysis of 16,515 HTA reports across 83 HTA bodies spanning 33 countries, the proportion of records incorporating RWE as part of the submission has risen from just 6% in 2011 to 39% in 2021.
- Increases in use have been accompanied by papers showing the use of RWE to emulate RCTs with comparable results, while statements and guidance made by organizations such as the U.S. FDA reflect increasing acceptance by HTA and regulatory bodies.
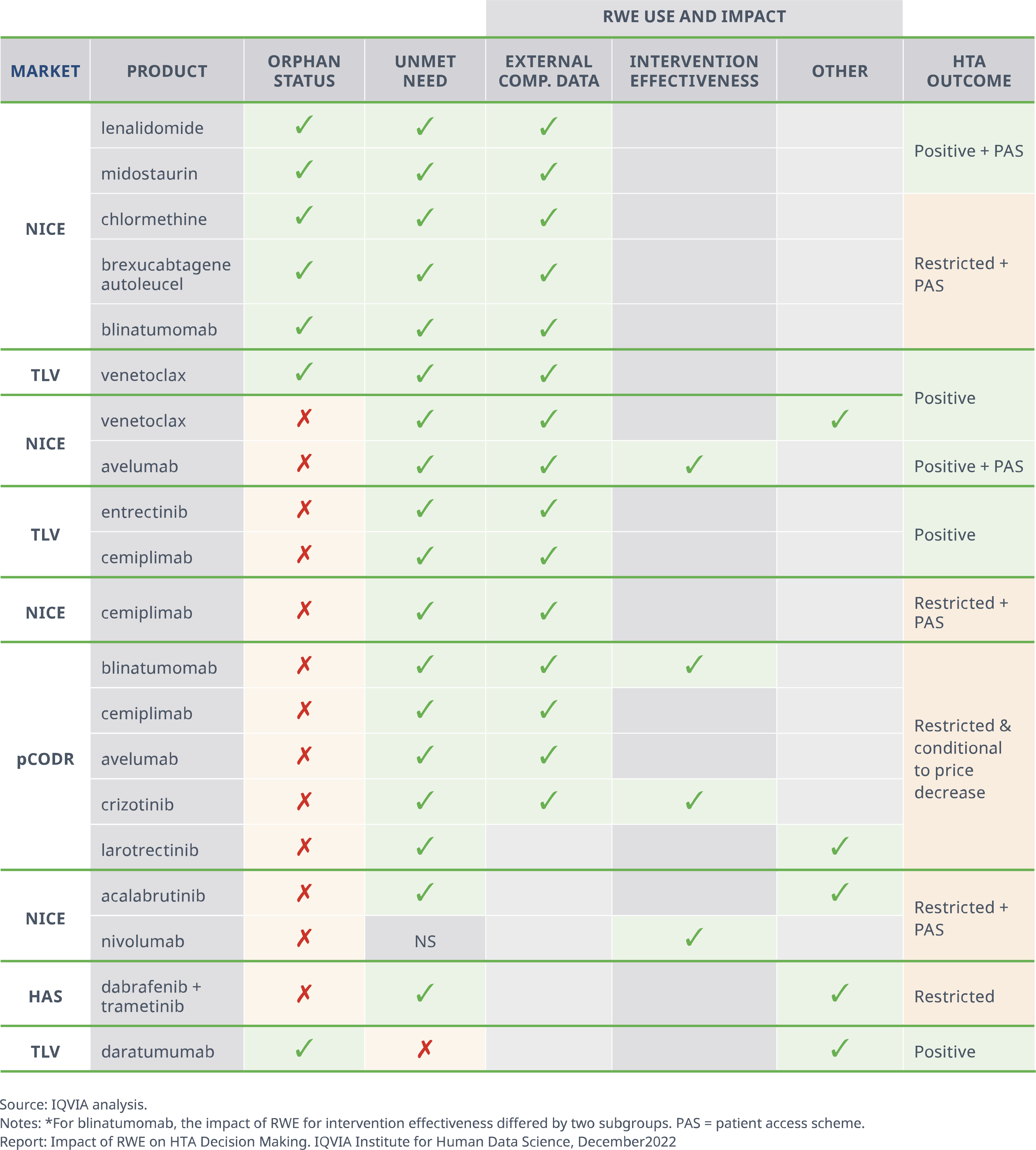
- In three quarters of the examples in which RWE was seen to have a positive impact on HTA, the RWE provided external comparator data for the standard of care (SoC), namely in terms of effectiveness or real-world use.
- One notable example is the review of cemiplimab by the TLV, where the pivotal trial lacked a comparator arm and RWE provided data on BSC and platinum-based chemotherapy enabling cemiplimab’s advantages to be demonstrated.
- In the remaining quarter, RWE was used to provide data on intervention effectiveness, the validation of surrogate endpoints, and patient characteristics.
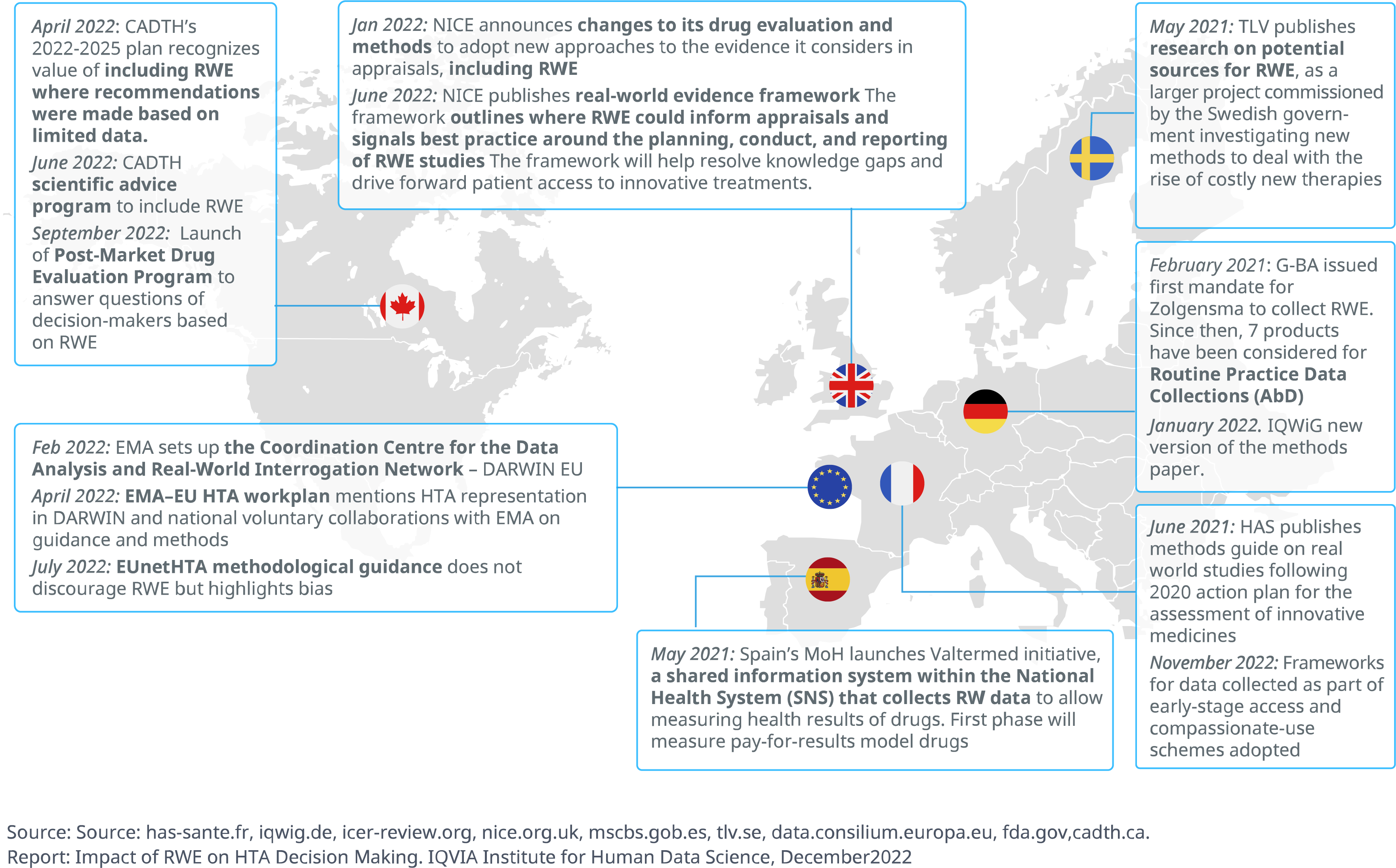
- At the national level, momentum is gathering to formalize the role of RWE in HTA, with several initiatives already having produced tangible results.
- France’s HAS published guidelines on the use of real-world studies in the assessment of medicinal products and devices in June 2021; in 2020, Canada’s CanREValue initiative developed a framework for the generation and use of RWE for cancer drug funding decisions; in Germany, which has historically been cautious in RWE adoption, the 2019 GSAV bill has opened the door to greater patient data registry use by allowing for the GBA to request data collection for orphan drugs that lack comparative data.

Copyright © 2023 IQVIA Holdings Inc. and its affiliates. All rights reserved. Privacy Statement
