Advancing Diversity in Clinical Development through Cross-Stakeholder Commitment and Action
An update on progress and results
The call for achieving representative diversity in clinical trials and development programs is not new — and indeed dates back more than five decades in the United States — but has been amplified over the course of the COVID-19 pandemic as the realities of disparities in health outcomes among diverse populations have become increasingly evident. At the same time, there has been a striking increase in the level of visibility, attention, commitment, and action by multiple stakeholders to address this issue.
The purpose of the research incorporated in this report is to measure the progress that has been made in increasing participation by racial and ethnic groups – with a principal focus on Black/African Americans and Hispanics – in clinical trials. We recognize that “diversity” can be framed in many different ways beyond race and ethnicity, and that there are limitations to what can be measured as well as time lags between action taken and results reported. Nevertheless, we believe that looking at the most recent available information provides a useful evidence base against which future progress can be measured.
We also highlight in this report where we see tangible progress being made, lessons learned and applied, and a positive shift among the many stakeholders involved in pursuing improvements in clinical research representativeness
Please submit the below form to download
Some key findings:
- Disparities in outcomes across different patient groups – defined based on race, ethnicity, gender, age, socio-economic factors, and other factors – are significant and can be attributed to many intersectional drivers related to healthcare awareness, access and delivery, in addition to factors related to genetics
- While access and delivery of care are major factors driving disparities in outcomes, a comprehensive clinical development program that addresses diversity and ranges from pre-clinical and exploratory studies through randomized confirmatory trials for safety and efficacy through to post-approval real-world studies can advance understanding of optimal patient care, expand access to care, bring earlier insights, and accelerate innovation
- Stakeholders in the U.S. have been focused on improving diversity in clinical trials for at least 30 years and a dramatic increase in reporting of diversity data has occurred over the past five years
- Based on race/ethnicity data reported, both Black/African American and Hispanic participation in trials fall short of 2020 U.S. demographics levels of 13.6% and 18.9% respectively
- Each stakeholder group has made significant commitments to support and enable clinical trials, and increasing clarity around what needs to be done and examples of progress and success cases provide important building blocks for future progress
- Success cases from sites that consistently over-recruit study participants relative to their demographics highlight some of the critical factors to engage under-represented populations in the clinical trial process
Other findings:
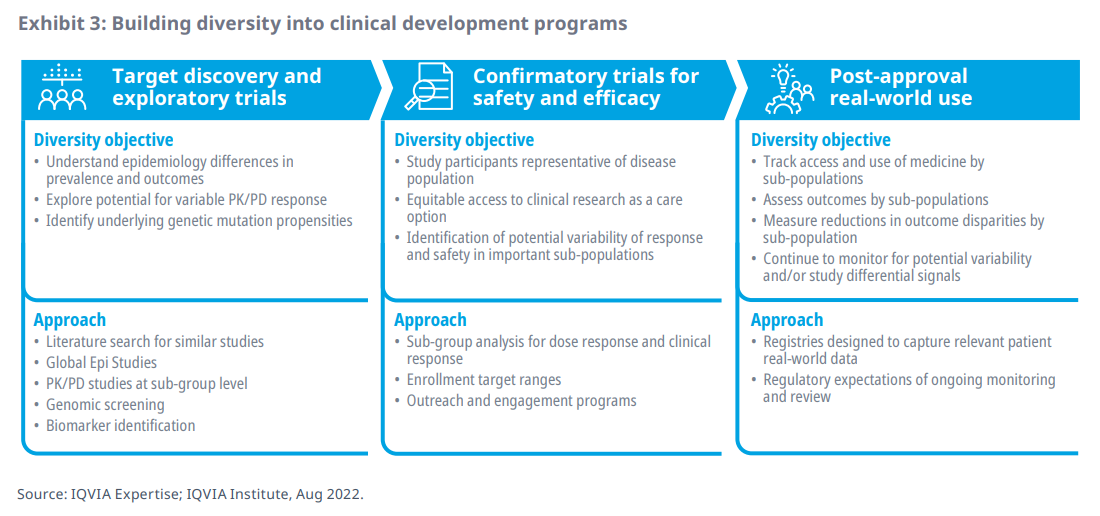
- Building an understanding of potential safety and efficacy variability across patient sub-groups requires comprehensive focus across the entire clinical development program of a new therapy– from target discovery and exploratory trials through confirmational clinical trials to study in post-approval real world data collection
- A key clinical development program goal should be to build a solid evidence base over time that increases confidence that if any clinically meaningful differences in safety and efficacy between sub-population responses do exist, they are identified and explored as early as possible and are factored into each stage of clinical trial planning
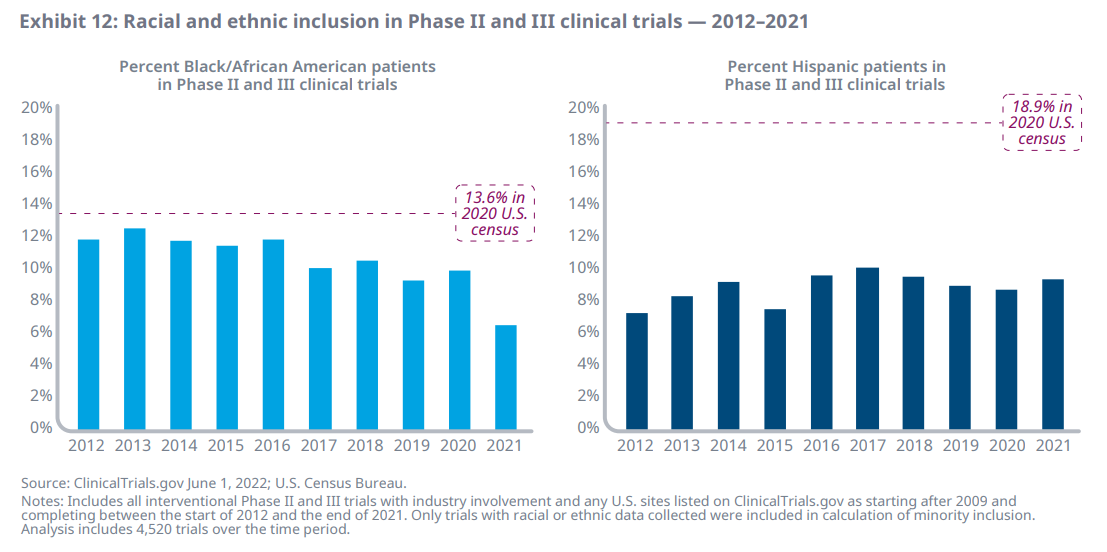
- Analysis of ClinicalTrials.gov data shows that relative racial and ethnic inclusion on clinical trials has remained steady or has decreased slightly over the past decade for interventional Phase II and III trials with industry involvement and at least one U.S. site
- Analysis of both Black/African American participation and Hispanic participation shows trials, on average, falling short of the 2020 U.S. demographics; specifically, Black/African American participation has been declining over the past decade – including a 15% drop in 2017 from a 2013 peak of 12.3% and a 35% decrease in 2020–2021 from 9.8% to 6.5%
- Hispanic inclusiveness varied over the timeframe, ranging from 7.4% in 2015 to a high of 9.9% in 2017
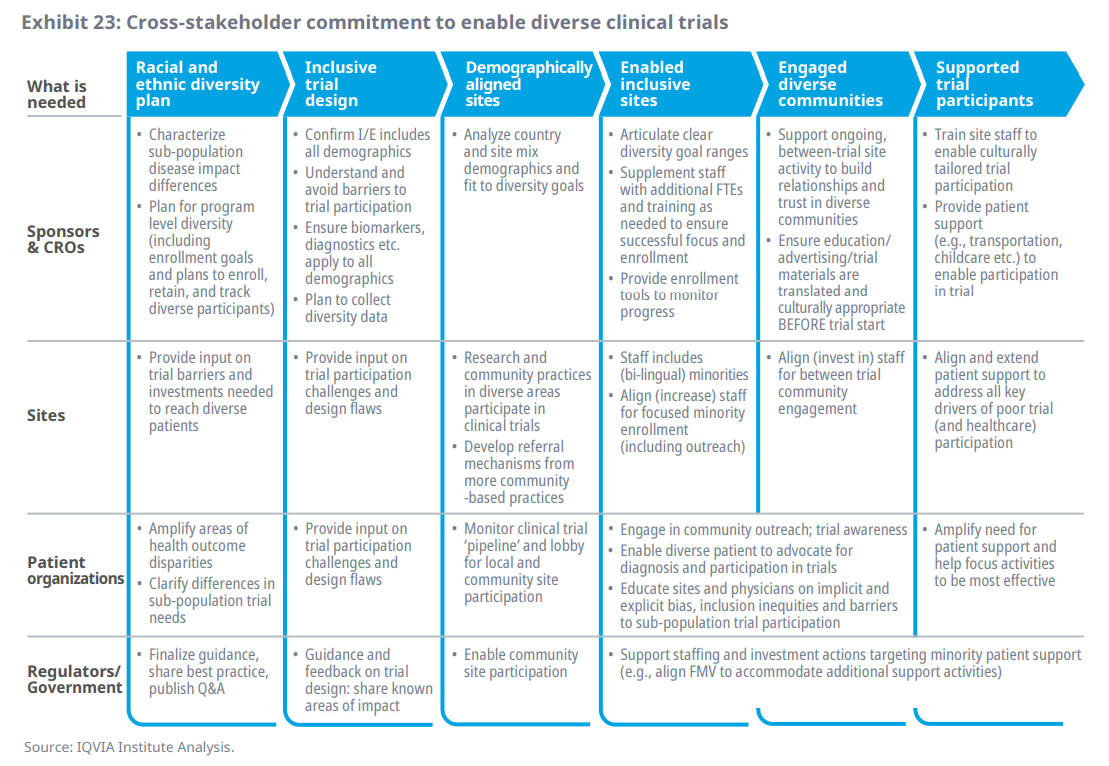
- Recent developments, including the drastic COVID outcome disparities seen in early 2020, along with a general ‘racial reckoning’ in the U.S. and elsewhere, has fueled a sense of urgency and has shifted conversations from the policy arena to the operational level
- With increasing focus on improving clinical research diversity and progress on trial results transparency, the healthcare ecosystem is poised to make appreciable gains in clinical research inclusivity; at the same time, examples of progress toward more inclusive clinical development are being demonstrated across stakeholders, and an integrated model of action has started to emerge
- Consideration of each stakeholder’s role, objectives, and examples of progress sets the stage for building more diverse clinical programs going forward; this includes sponsors and their partners, sites, patients, and regulators, who all play a critical part in advancing a more equitable clinical innovation system

Copyright © 2023 IQVIA Holdings Inc. and its affiliates. All rights reserved. Privacy Statement
