Shared Savings Programs in Europe
Lessons for the United States
Acceptance and use of biosimilars has been rising in the U.S. Biosimilars can enhance the sustainability of the overall healthcare system through savings and increased access. Currently, there are policies under discussion in the U.S. to improve access to biosimilars, and some of these policies include shared savings programs (also known as benefit sharing or gain sharing in other countries). Shared savings programs have been implemented in a number of countries in Europe. While contexts across countries vary, the experience of such programs in other countries can provide lessons for the U.S.
In this report, five case studies of shared savings/benefit sharing programs from across Europe are examined using secondary research, expert interviews and IQVIA data. The background to setting up a program is provided for each case study along with the structure of the program and results as stated in secondary literature and as per IQVIA data. Lessons for the U.S. are summarized based on these case studies.
Please submit the below form to download
Some key findings from one case study in Ireland:
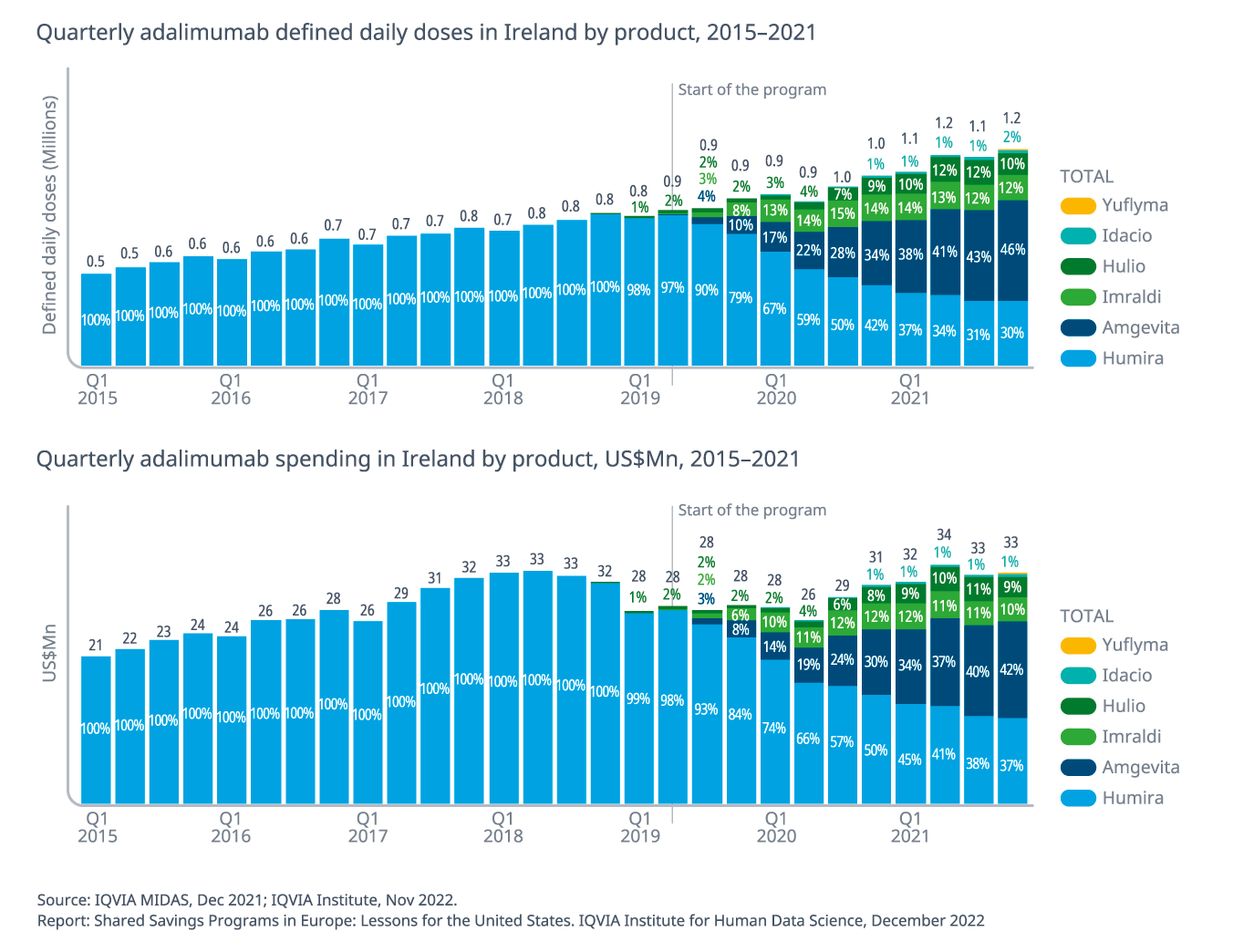
- Between Q2 2019 and Q4 2021, the adalimumab biosimilar share increased from 2% to 70%.
- Adalimumab has seen a volume increase of 50% since introduction of the program while costs have increased at a slower rate by 19% (at list price level).
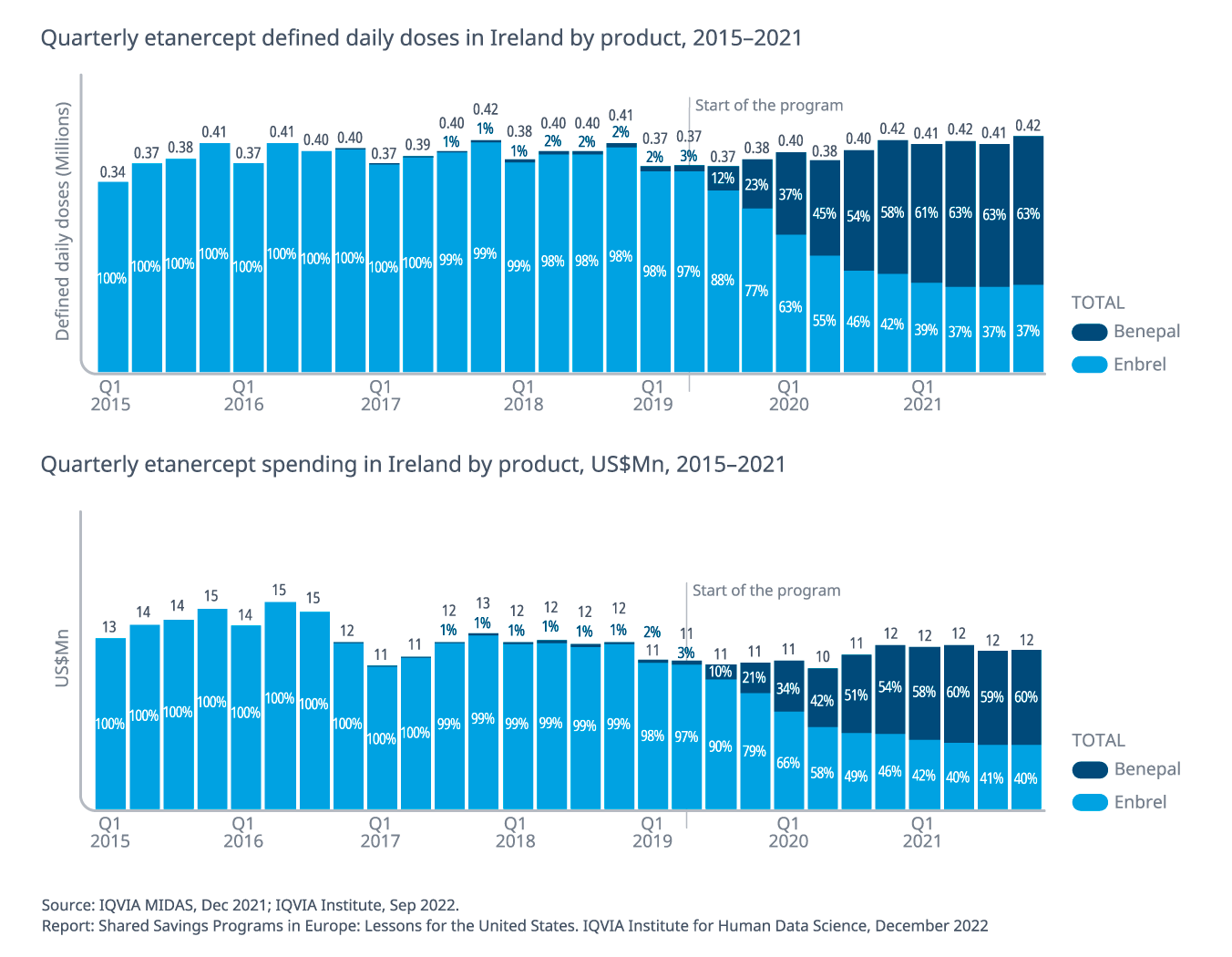
- Between Q2 2019 and Q4 2021, the etanercept biosimilar share increased from 3% to 63%.
- Etanercept’s volume has increased by 14% while costs have increased by 7% (at list price level).
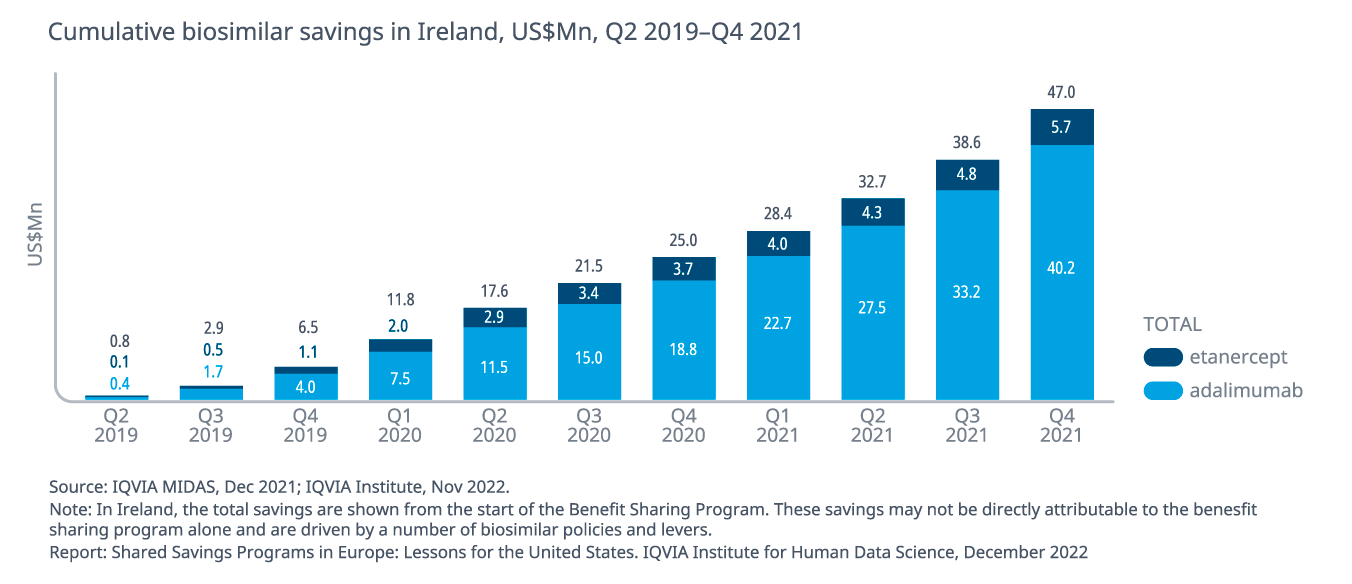
- Utilizing the IQVIA data to estimate savings between Q2 2019 and Q4 2021, the total savings due to biosimilar use (at a list price level) for adalimumab and etanercept are estimated at $47 million.
- Although the savings and usage may not be directly attributable to any one policy, the benefit sharing program has been viewed as a success in Ireland, especially given the low uptake of biosimilars prior to 2019.

Copyright © 2023 IQVIA Holdings Inc. and its affiliates. All rights reserved. Privacy Statement
